What allows valence electrons in metals to move throughout the metal?
A towering skyscraper bends under a strong gust of current of air, but information technology doesn't snap. That's ane advantage metals can offer. Being malleable (MAAL-ee-ah-bul), metals tin be hammered into sheets without shattering. Considering they're ductile (DUK-tul), they can exist easily pulled and stretched into wires without snapping. Metals also can comport electricity.
But non all metals are every bit prized. Although more than three-fourths of the 118 elements on the periodic table are metals, nosotros arts and crafts tools from only a few of these. That'southward because familiar metals, such as iron and silver, are special. For i matter, they're easier to discover than other metals. (Although most known elements are metals, they are adequately uncommon in nature.)
Familiar metals too are less reactive than most other metals. Reactivity refers to how easily a substance reacts chemically with other substances. Low-reactivity metals are safer to handle than loftier-reactivity ones. Pure argent is so safe that we utilise it for jewelry and flatware. Just pure sodium, also a metal, is so reactive it explodes on contact with water!
Adept matter, and then, that pure sodium never occurs naturally. Instead, we find it after it has already bonded chemically with one or more other elements. Sodium chloride, or table salt, is a common example. And that highlights another reason low-reactivity metals are so useful. They often occur naturally in forms like shooting fish in a barrel to work with. For case, silver can be mined as pure silver. Only if we wanted pure sodium, we'd need a way to separate information technology from one of the chemicals to which it had bonded. That can be hard to do.
Sometimes, a special projection — such equally the $x-billion James Webb Space Telescope — may call for a fairly rare metallic. After 25 years in development, the telescope launched on Christmas morning in 2021. For this center in the sky, NASA chose the rare metallic beryllium (Beh-RIL-ee-um) to make its honeycomb of gold-plated mirrors. Beryllium is super lightweight. That makes it easy to launch into infinite. Glucinium also holds its shape in frigid temperatures. When rocketed from Earth'southward relatively lukewarm air to the cryogenic (super-cold) temperatures of space, metals contract and bend. Since telescopes work by reflecting light, whatever tiny change in their shape could ruin a telescope's images. But beryllium remains more stable than most metals during such abrupt temperature changes.

Metal vs. nonmetal: What'south the departure?
If information technology's able, an cantlet will steal electrons from a neighboring cantlet. It won't steal all of them, only just enough to exist stable. Unlike elements accept different numbers of electrons that — at least in theory — can be stolen by a neighbor. These are called valence (VAY-lents) electrons. They are the outer, orbiting electrons that can get office of chemical bonds.
Metallic atoms differ from nonmetal ones in how well they steal valence electrons from other atoms. One might say that metals are bad thieves. Instead of capturing a neighbour'due south electrons, they usually give up their own. This tendency to lose electrons is described equally their "metallic character."
Nonmetallic elements, therefore, have a low metallic character. Among these nonmetals are carbon, oxygen and nitrogen. When it comes to electron thieves, nonmetals are the all-time. King of those nonmetals is fluorine. When it comes to electron-stealing, fluorine's a downright swell. So it's highly reactive.
Metals practice desire electrons, but only weakly. In many ways, withal, this weakness turns out to exist metals' strength. Their malleability (bendiness), ductility (stretchiness) and conductivity come from the tendency of these elements to lose electrons.
Metals can fifty-fifty class a special bond
Most chemical bonds occur as atoms fight over electrons. A metallic bond occurs when two metal atoms bond. Neither of them seems to care much which atom ends up with the actress electrons.
Contrast this with an ionic bond. That occurs when a metal (like sodium) bonds with a nonmetal (such as chlorine). Due to big differences in their metallic character, the nonmetal steals electrons from the metal atom and keeps those electrons.
Chemical bonding occurs after the theft. The metal and nonmetal remain stuck together because they now accept reverse charges. Both atoms started with a neutral accuse. Once the nonmetal gained an electron, it became negative. (That's because electrons take a negative accuse.) Merely the metal lost an electron. That left the metal with an overall positive charge. Observe that the metal isn't positive because information technology gained a positive accuse. It'due south positive considering it lost a negative ane. These oppositely charged atoms, at present called ions, attract each other and stick together.
Metal bonds likewise differ from the covalent bonds that form when 2 nonmetals join up. In that location, both nonmetals attempt to wrestle electrons from the other. But sinceboth have stiff claims on the other'south electrons, they both fail. The nonmetals finish upwardly locked in a perpetual tug-of-war. Because neither cantlet "wins," these atoms are described equally "sharing" their electrons.
But when two metallic atoms bump into each other, they don't fight for electrons. Neither really wants the electrons badly enough. When many metal atoms end upwardly stuck together, as in a piece of metal, their electrons move from i atom to another to some other to another. Scientists describe these electrons every bit "delocalized."
Delocalized electrons explain why metals behave electricity. Later on all, electricity is merely the movement of electrons. One model used to explain metallic bonds envisions metal atoms as though they float through an ocean of electrons.
Delocalized electrons don't just explain metals' electrical conductivity. They also explain metals' malleability and ductility.Metal bonds let metal atoms to movement around inside their electron bounding main, all the same even so remain bonded. That would never be possible if they were locked rigidly together with covalent or ionic bonds.
The atoms in some metals move around more easily than others. Metals with hands moveable atoms are likewise malleable to use in tool-making. They're as well soft. Sodium metal is one such example. Sodium is malleable enough to cut with a spatula. Most of our really useful metals, especially those used for making tools, are difficult enough to keep their shape. Take iron, for instance. To forge an iron sword, a blacksmith must reposition bajillions of iron atoms. And that's no easy task.
So, and so, how do blacksmiths exercise information technology? Keep in mind that atoms never sit still. They motion and shake. And hot atoms shake more than cold ones, giving hot atoms more liberty. In molten metallic, the bonds betwixt atoms weaken a lot. So a blacksmith will boost a forge's rut to upwardly of 1,600º Celsius (2,900º Fahrenheit). This intense fever weakens those metallic bonds, allowing the blacksmith to hammer the metal into shape.
Once the new sword cools, its atoms deadening and the metal bonds re-secure.
Alloys and a truly Titanic lesson
Jewelry well-nigh never contains pure golden. Information technology would simply be likewise soft. So metal-workers mix gold with other metals, often copper, to make information technology less malleable. Such mixed metals are called alloys. Alloys can also be metals mixed with a tiny dash of some nonmetal.
Carbon steel is an atomic number 26 alloy made with a pinch of carbon. Skyscrapers and bridges are made from lots of carbon steel. So are many kitchen knives and screwdrivers. The carbon in carbon steel reinforces the iron, making it harder. But increasing a metal'south hardness also drops its malleability.
In fact, as well much carbon will make steel brittle. And as metals get cold, this trouble only worsens. In April 1912, the cruise transport Titanic struck an iceberg on its maiden voyage and sank. More than i,500 people died. Studies on the ship'south gashed hull advise that the Titanic's steel became brittle in the Atlantic's frigid waters. The Titanic's steel alloy became breakable at 32 ºC (xc ºF). The night Titanic sank, the water was only –2 ºC (28 ºF).
Engineers today would use a dissimilar steel. For example, a mod steel-type called ASTM A36 can handle –27 ºC (–17 ºF) earlier becoming every bit brittle as the Titanic's had. The difference is in the alloy. The Titanic's steel "had a lot more than sulfur content and a lot less manganese," explains Andrew Falkowski. He's a materials engineer in Salt Lake City, Utah. He too co-hosts the Materialism Podcast, a prove well-nigh materials science.
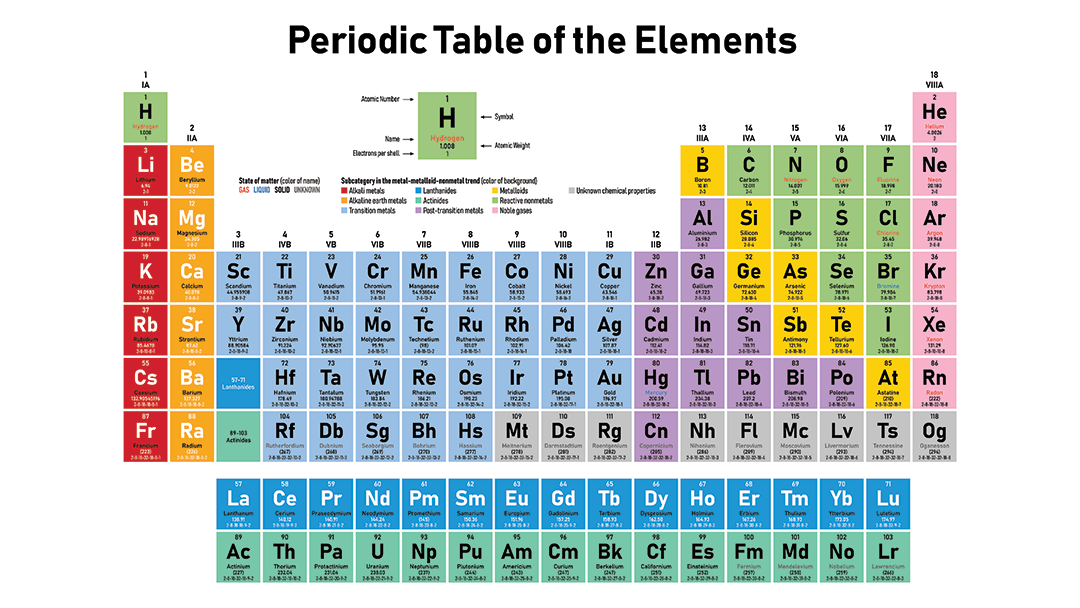
The periodic table separates metals from nonmetals
You can tell how metallic any element is just past finding it on the periodic table. Metal graphic symbol exists on a spectrum. Elements fall somewhere betwixt the least metal — fluorine — and the most metallic — cesium (or francium, if yous include lab-made elements). And so in terms of metallic character, cesium and fluorine are polar opposites. Fluorine is the most reactive nonmetal in being. And cesium is the most reactive metal. If these ii ever meet, they'll burst into intense white burn down.
Detect these elements on a periodic table, and you'll notice something. They're on opposite sides. That's no coincidence.
Nonmetals occupy the upper right of the archetype periodic table, including the entire far-right column. In that location's i exception. Hydrogen is the but nonmetal that is non grouped with the nonmetals. Hydrogen is weird, mainly because its atoms are then tiny.
Metals occupy everywhereexcept the upper right. As you lot motion away from the nonmetals, metallic graphic symbol increases. It increases as you move from correct to left and too as you motion down.
Similar types of metals grouping together on the periodic table. For case, the far-left column contains sodium and the other and so-called alkali metals. All of these react violently with nonmetals. The elements that lie betwixt metals and nonmetals are called metalloids or semi-metals. They have backdrop of both metals and nonmetals. Arsenic and silicon are examples. In the middle are what are known every bit transition metals. That's where most familiar metals live, such as gold, silver and copper.
Source: https://www.sciencenewsforstudents.org/article/explainer-what-is-a-metal

0 Response to "What allows valence electrons in metals to move throughout the metal?"
Postar um comentário